Method for express analysis of inhibition of living protein molecules invented at JINR
Media, 08 August 2022
Currently, the most accurate and informative method for studying the structure of complex protein molecules is the measurement of shifts in the peaks of nuclear magnetic resonance (NMR) of these molecules. Today, the Joint Institute for Nuclear Research has all the conditions for creating an NMR spectrometer. Thanks to the new JINR invention, such a spectrometer could register radiation-induced damages to molecules in real-time mode, which will increase the informative value of studies.
 According to the author of the invention, a senior researcher of the Laboratory of Nuclear Problems, Candidate of Physics and Mathematics Sergey Dolya, NMR spectroscopy has unique capabilities for research in the field of protein molecules, which are capable of, for example, forming polymers from monomers, operating as if according to a given algorithm. However, in modern spectrometers, the effect of the radiation absorbed dose on the molecule is recorded after their irradiation, which does not allow seeing the injury pattern of protein molecules by radiation directly during their irradiation. The new method for express analysis of the structure of living protein molecules allows obtaining information about them in a dynamic mode, during radiation exposure.
According to the author of the invention, a senior researcher of the Laboratory of Nuclear Problems, Candidate of Physics and Mathematics Sergey Dolya, NMR spectroscopy has unique capabilities for research in the field of protein molecules, which are capable of, for example, forming polymers from monomers, operating as if according to a given algorithm. However, in modern spectrometers, the effect of the radiation absorbed dose on the molecule is recorded after their irradiation, which does not allow seeing the injury pattern of protein molecules by radiation directly during their irradiation. The new method for express analysis of the structure of living protein molecules allows obtaining information about them in a dynamic mode, during radiation exposure.
The essence of the new invention lies in the fact that when performing NMR spectroscopy of living protein molecules, it is possible to simultaneously irradiate them and compare the rate of chemical reaction yield with a control sample. According to measurement results, you can obtain information about the effect of the radiation absorbed dose on the rate of the biochemical reaction. The technical result is to provide the ability to carry out and determine chemical shifts in living protein molecules quickly, directly in the course of a chemical reaction under the effect of radiation exposure of protein molecules using NMR spectroscopy.
“We can observe these molecules directly during their operation and under irradiation. NMR spectrometry shows which part of the molecule we have damaged with our emitter and how this damage has affected the performance of the molecule,” Sergey Dolya comments on the invention. “Using the new method, we will be able to see what other researchers may have missed: the molecules were first irradiated, and then the shifts of NMR peaks were measured. But during this time the structure of the molecule could have changed. Moreover, this method allows you to monitor the performance of the molecule “live”, directly during irradiation.”
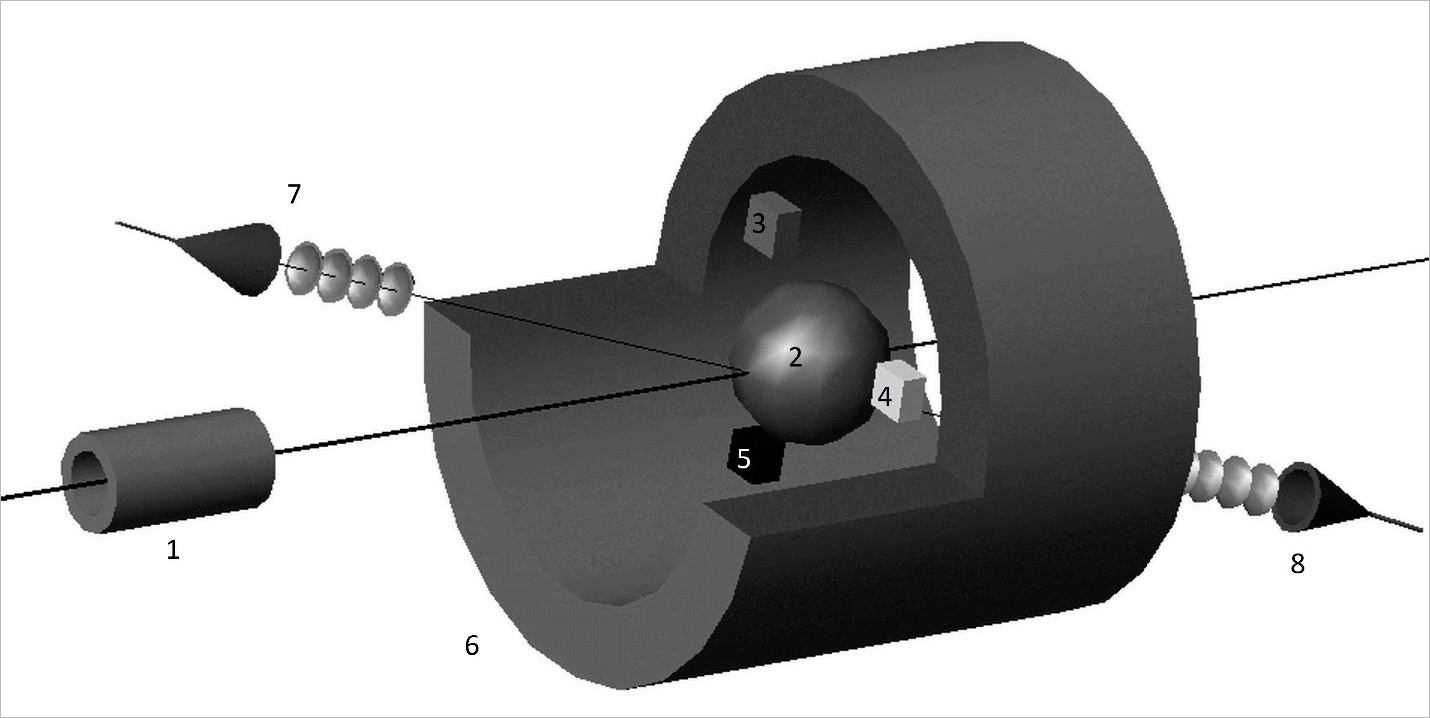 Scheme of facility for express analysis of inhibition of living protein molecules. (1) — radiation source, (2) — test sample, (3-5) — concentration meters of starting substance and reaction products: glucose meter (3), alcohol tester (4), gas analyser (5); (6) — superconducting solenoid, (7) — narrowband radiation generator, (8) — receiver tuned to frequency of generator
Scheme of facility for express analysis of inhibition of living protein molecules. (1) — radiation source, (2) — test sample, (3-5) — concentration meters of starting substance and reaction products: glucose meter (3), alcohol tester (4), gas analyser (5); (6) — superconducting solenoid, (7) — narrowband radiation generator, (8) — receiver tuned to frequency of generator
The scientist added that radiation sources could be completely different: both electron, proton, and neutron beams are suitable for research. “If you irradiate molecules with neutrons, there will be one effect. If you irradiate them with heavy ions, there will be another effect. This is because the DNA molecule consists of two strands that are twisted into a coil. Heavy ions immediately interrupt two strands of the coil. Gamma quanta damage only one. As a result, the coil can easily recover after irradiation with gamma rays having a copy of itself. However, it will be more difficult for it to recover after irradiation with heavy ions. The new method for analysis will make it possible to track by the rate of the biochemical reaction how radiation damage affects the performance of the molecule, which damages turn out to be fatal, and which of them allow the molecule to recover and for how long,” Sergey Dolya said.
There are a lot of examples of molecules that can be subjected to such an analysis. Enzymes, i.e. biological catalysts, are one of them. According to Sergey Dolya, their effect can be compared to a game of Tetris. In Tetris, as you know, figures from squares of different shapes fall on the field. If they fall arbitrarily, they can very rarely fill the whole row. But if a person uses a joystick to rotate them in the right direction, then a large number of rows are filled in this way. The catalyst works in the same way: without participating in the reaction, it turns the molecules in the right direction and connects them so that the reaction rate increases. At the same time, for biological objects, the reaction rate increases hundreds of thousands of times.
There is no NMR spectrometer at JINR yet, but there are prerequisites for creating it. This requires a strong superconducting magnet with a uniform magnetic field. A hole should be provided in the centre of the magnet through which the test sample will be introduced into the magnetic field.
The shift of the peak of the NMR resonance directly depends on the size of the magnetic field. If the magnetic field is non-uniform, then the size of the resonance shift will be different for its different regions, and the peak will be blurred. Thus, the accuracy of the spectrometer readings directly depends on the accuracy of the magnet manufacturing. “JINR is just good at calculating and manufacturing such magnets,” Sergey Dolya explained.
The magnet for NMR spectroscopy is similar in structure to the magnet for the proton medical cyclotron MSC-230 being developed at JINR. Most probably, an intermediate model magnet will be manufactured for the cyclotron at first, which can later be used for the needs of spectroscopy of protein molecules using nuclear magnetic resonance.
The patent for the invention “Method for express analysis of the structure of living protein molecules” was obtained by the Joint Institute for Nuclear Research on 18 July 2022.
