JINR scientists tested bakery yeast’s ability to extract precious metals from wastewater
Media, 29 June 2023
Performing experiment with silver-containing wastewater and ordinary baker’s yeast Saccharomyces cerevisiae, the group of scientists from the Sector of Neutron Activation Analysis and Applied Research of the Frank Laboratory of Neutron Physics of JINR confirmed the amazing ability of yeast to adsorb metal ions. The researchers were able to achieve 100% silver removal from wastewater. They believe that this type of yeast can become a promising biosorbent, which is not only safe but also cost-effective.
Silver compounds due to their high electrical and thermal conductivity, plasticity, corrosion resistance and, especially, antimicrobial properties are widely used in medicine, chemical industry, electroplating, jewelry production, photography, etc. However, despite all the benefits of this precious metal, it is highly toxic to living organisms. Therefore, studies related to environment bioremediation, including treatment of wastewater containing silver, are getting more relevant.
Currently, such methods as chemical precipitation, adsorption, ion exchange, membrane separation, ultrafiltration, reverse osmosis and electrolysis are used to remove silver ions from wastewater. These techniques allow extracting from 60 to 99% of silver ions from wastewater. At the same time, the majority of them, in addition to high cost and energy consumption, have a number of other disadvantages, for example, high requirements for reagents and formation of toxic sludge that require special processing and storage conditions, and some of the methods are time-consuming.
Biosorption, namely, the use of microorganisms for wastewater treatment, is considered one of the most promising technologies due to the low cost and availability of biosorbents, the possibility of their reuse, high sorption capacity, and even the possibility of selective metal ions recovery. Biosorption is based on physicochemical interactions between the metal ion and the functional groups present on the microorganism cell surface.
JINR scientists in earlier studies have already proven the effectiveness of using yeast Saccharomyces cerevisiae for metal removal from wastewater. Thus, while working with synthetic zinc-containing effluents containing nickel, copper, strontium, and barium ions in addition to zinc, the influence of a number of parameters, such as sorption time, temperature, initial zinc concentration and pH, on the biosorbent removal efficiency was studied. According to the obtained results, in 15-45 minutes, necessary for the effective removal of metals, and at optimal pH values in the range of 3.0-6.0, it was possible to remove 45-100% of metal ions, depending on the metal, from wastewater.
In the course of the following experiments, scientists similarly studied the interaction of yeast and silver ions present in wastewater. Three different effluents were simulated: one of them contained only silver, the second one contained silver and copper, and the third one had silver, copper, nickel and zinc. Dry yeast biomass was added to each of the solutions, and then the effect of various parameters (pH, contact time, temperature, and silver concentration) on the metal removal by yeast biomass was studied. To assess the efficiency of metal ions uptake by biosorbents, the scientists used neutron activation analysis at the IBR-2 Pulsed Reactor at FLNP, a sensitive method for the qualitative and quantitative determination of elements. Atomic absorption spectrometry was used to determine the uptake of copper.
 Employees of the Sector of Neutron Activation Analysis and Applied Research, FLNP JINR. Inga Zinicovscaia and Dmitrii Grozdov
Employees of the Sector of Neutron Activation Analysis and Applied Research, FLNP JINR. Inga Zinicovscaia and Dmitrii Grozdov
 Employee of the Sector of Neutron Activation Analysis and Applied Research, FLNP JINR. Nikita Yushin
Employee of the Sector of Neutron Activation Analysis and Applied Research, FLNP JINR. Nikita Yushin
“The process of metal removal was fast and pH dependent. The maximum removal of silver and copper ions in all the systems was achieved at pH 3.0, of nickel ions – at pH 6, and of zinc ions – at the pH range 3-6,” Inga Zinicovscaia, Head of the Sector of Neutron Activation Analysis and Applied Research at FLNP JINR, says. “With the increase of the adsorption time from five to forty-five minutes, the sorption capacity of the yeast biomass increased sharply. At the same time, it should be noted that the most noticeable increase of silver ions removal occurred in the first five minutes of the sorbent interaction with the sorbate. The efficiency of silver ions removal ranged from 97 to 100%”.
She also noted that the yeast biomass retained a high sorption capacity for silver ions even at high concentrations of metal ions in solution. For example, at silver concentrations in solution up to 100 mg/L microorganisms were able to remove almost completely metal ions. The efficiency of yeast biomass to remove silver ions, depending on the temperature of the solution, differed in systems under study, but the same effect was observed: with an increase in temperature the adsorption of metals onto biomass increased. Scientists explain this by a decrease in the viscosity of the solution and an increase in the mobility of metal ions in the solution.
“The rough surface of yeast cells facilitates the access of metal ions to active sites, which consequently increase the efficiency of the biosorption. Therefore, it can be assumed that the biosorption of metals occurs on the cell surface. We consider that sorption occurs due to surface complexation, coordination or ion exchange, – said Inga Zinicovscaia. “The results of neutron activation analysis confirmed the significance of the ion exchange process in the removal of metal ions.”
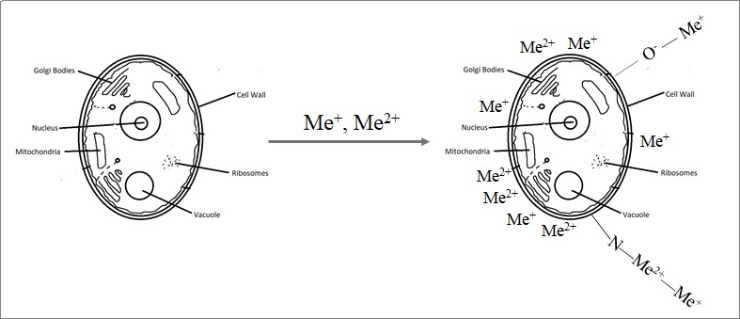 Metal ions biosorption onto yeast biomass
Metal ions biosorption onto yeast biomass
It is noteworthy that the yeast biomass retained a high adsorption capacity during three sorption–desorption cycles. The authors conclude that this allows it to be used for repeated removal of metal ions. This property, as well as the high biosorption capacity and safety of the yeast make it possible to produce an environmentally friendly and cheap biosorbent that is easy to handle and can be used to treat large volumes of wastewater.
“This is also promising from the point of view that using yeast it is possible to establish the recovery of silver from wastewater. This is especially important due to the fact that the world’s reserves of precious metals, including and silver are drying up, mines are getting poorer, and traditional methods for metal ions removal have their drawbacks. The way out of this situation may be the wider use of microbial technologies for the extraction of precious metals. They can be used for silver, gold, platinum, and even for rare earth elements recovery,” concluded Inga Zinicovscaia.
Publication
- Zinicovscaia I, Yushin N, Grozdov D, Rodlovskaya E, Khiem LH. Yeast—As Bioremediator of Silver-Containing Synthetic Effluents. Bioengineering. 2023; 10(4):398. https://doi.org/10.3390/bioengineering10040398