Study of the Mechanisms of Interaction of Coronaviruses with the Cell Membrane
Publications, 12 November 2020
We kindly invite you to read the article “Study of the Mechanisms of Interaction of Coronaviruses with the Cell Membrane” by D. Soloviov, M. Zhernenkov published in the 3rd issue 2020 of the bulletin “JINR News”.
Viruses are an extracellular life form that has its own genome. However, a prerequisite for the reproduction and spread of the virus is its penetration into human or animal cells. Therefore, viral pathogens are often the cause of epidemics that paralyze the usual way of life of a large number of people. The recent SARS-CoV-2 coronavirus pandemic has once again highlighted the importance of developing scientific approaches to prevent the spread of viral infections. In light of this, scientists from the Frank Laboratory of Neutron Physics (JINR) in collaboration with their colleagues from the Brookhaven National Laboratory (USA) have launched comprehensive research in this area. It is planned to use the advantages of neutron and synchrotron scattering methods to identify the structural and dynamic characteristics of the cell membrane containing the transmembrane receptor protein ACE2 when interacting with the viral pathogen SARS-CoV-2 (Fig. 1). Within the framework of the studies, experiments will be conducted at the world’s best synchrotron radiation sources: ESRF (France), SPring-8 (Japan), APS (USA), as well as using the neutron scattering instruments of the IBR-2 reactor
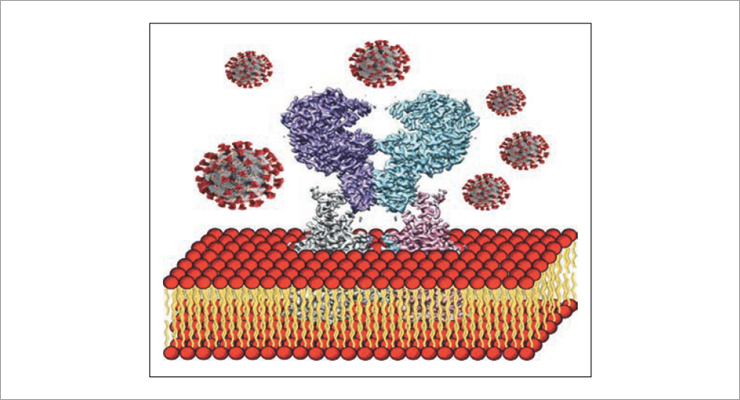 Fig. 1. Schematic illustration of the interaction of the lipid membrane containing the ACE2 receptor protein and SARS-CoV-2
Fig. 1. Schematic illustration of the interaction of the lipid membrane containing the ACE2 receptor protein and SARS-CoV-2
It is worth noting that the planned investigations are a logical continuation of the previous studies of lipid membranes. So, for example, in 2014, collective vibrations of lipid molecules in single-component membranes of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were studied using high-resolution inelastic X-ray scattering [1]. Here, for the first time, the existence of a transverse acoustic phonon mode in the lipid membrane was proved experimentally. It was also shown that when the lipid is heated above the phase transition temperature Tm, the above phonon mode exhibits a gap in the region of small values of the scattering vector Q (Fig.2).
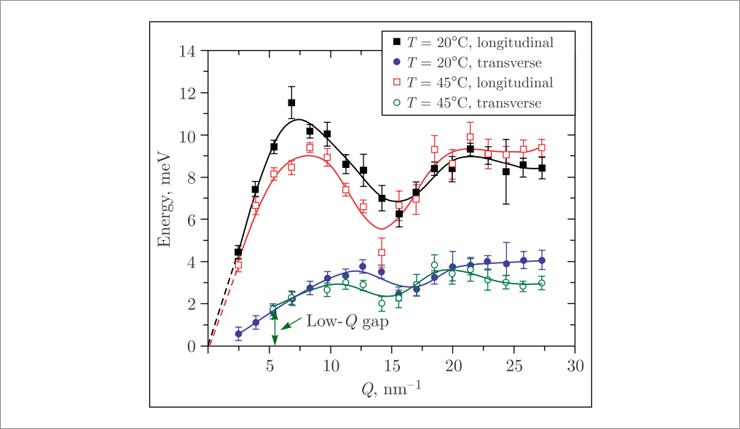 Fig. 2. Dispersion curves of longitudinal and transverse acoustic phonon modes propagating in the DPPC lipid membrane
Fig. 2. Dispersion curves of longitudinal and transverse acoustic phonon modes propagating in the DPPC lipid membrane
The observed gap is associated with diffusion and relaxation processes occurring in the lipid membrane and is a direct evidence of short-lived (on the order of several picoseconds) spontaneous occurrence of nanometer-sized lipid clusters surrounded by voids in the membrane (Fig.3). These pores determine the mechanism of passive transport of solutes through the lipid membrane.
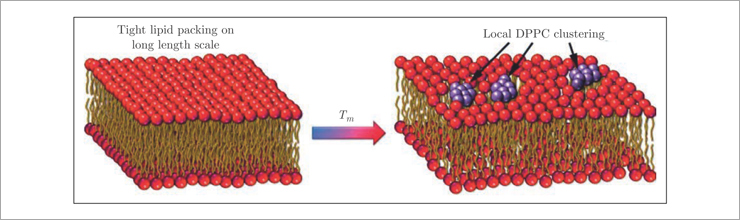 Fig. 3. Spontaneous formation of pores in the lipid membrane
Fig. 3. Spontaneous formation of pores in the lipid membrane
The use of inelastic X-ray scattering and molecular dynamics simulations made it possible to study in detail the phase separation processes in more complex two- and three-component lipid membranes of DPPC–cholesterol and POPC/DOPC–DPPC–cholesterol [2]. The appearance of optical phonon modes in the dispersion curves of these systems (Fig. 4) suggests the existence of stable (on the picosecond time scale) functional lipid pairs of DPPC, POPC, DOPC and cholesterol molecules that oscillate in antiphase around their centers of mass (Fig. 5). Thus, the observed gap in the optical phonon modes is a consequence of the finite size of the regions (nanodomains) formed by these lipid pairs.
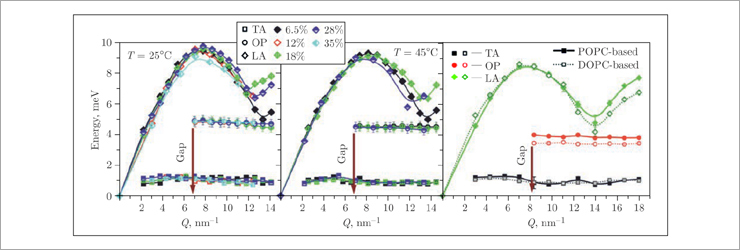 Fig.4. Dispersion curves for the binary DPPC–cholesterol lipid mixtures (left and middle) at different temperatures and mol% of cholesterol (see legend) and ternary POPC/DOPC–DPPC–cholesterol systems at 37°C (right). The phonon gap in the optical mode is indicated by the arrow
Fig.4. Dispersion curves for the binary DPPC–cholesterol lipid mixtures (left and middle) at different temperatures and mol% of cholesterol (see legend) and ternary POPC/DOPC–DPPC–cholesterol systems at 37°C (right). The phonon gap in the optical mode is indicated by the arrow
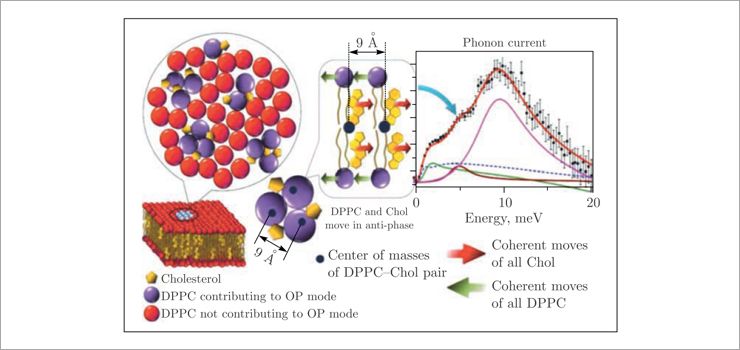 Fig. 5. Schematic view of the functional lipid pairs that nucleate into nanodomains. As a result of coherent antiphase oscillations of neighboring DPPC and cholesterol molecules about their static centers of mass (black circles), the optical phonon mode is confined within the nanodomain
Fig. 5. Schematic view of the functional lipid pairs that nucleate into nanodomains. As a result of coherent antiphase oscillations of neighboring DPPC and cholesterol molecules about their static centers of mass (black circles), the optical phonon mode is confined within the nanodomain
It is known that it is the properties of the lipid membrane that largely determine the set of biological functions of individual cell components, including membrane proteins. The ultrafast dynamics of functional lipid pairs that we observed is comparable on the time and energy scale with the processes of relaxation of transmembrane proteins. Therefore, the study of the structural and dynamic properties of lipid membranes in the presence of viral infection will help to understand the fundamental features of the interaction of the cell with the viral pathogen.
References
- Zhernenkov M., Bolmatov D., Soloviov D., Zhernenkov K., Toperverg B.P., Cunsolo A., Bosak A., Cai Y.Q. Revealing the Mechanism of Passive Transport in Lipid Bilayers via Phonon-Mediated Nanometre-Scale Density Fluctuations // Nat. Commun. 2016. V. 7. P.11575.
- Soloviov D., Cai Y.Q., Bolmatov D., Suvorov A., Zhernenkov K., Zav’yalov D., Bosak A., Uchiyama H., Zhernenkov M. Functional Lipid Pairs as Building Blocks of Phase-Separated Membranes // Proc. Nat. Acad. Sci. USA. 2020. V.117(9). P.4749‒4757.