Brewer’s yeast tackling heavy metals
Media, 09 March 2022
Scientists from the Laboratory of Neutron Physics JINR have found that ordinary brewer’s yeast can act as an effective sorbent for treatment of wastewater contaminated with heavy metals. The study has shown that this technique is also cost-effective and environmentally friendly.
Heavy metals are among the most dangerous environmental pollutants due to their high toxicity and ability to accumulate in the food chain. One of the important ways for heavy metals to enter the environment is the discharge of untreated or insufficiently treated industrial effluents into natural waters. Release of industrial effluents in the environment has a powerful technogenic impact on water and soil ecosystems, causing disturbances in the natural development of biogeocenoses. In this regard, today an important task is to develop environmentally friendly, cheap, and efficient methods of metal ions removal from industrial effluents. Traditionally used methods have a number of disadvantages, namely: the need to use chemicals, the production of large volumes of secondary waste, high cost and energy consumption. In addition, at low concentrations of metals in wastewater, these techniques show low removal efficiency.
Employees of the Sector of Neutron Activation Analysis and Applied Research of FLNP JINR have accumulated considerable experience in conducting research in the field of environmental chemistry. Various biological and composite sorbents, namely yeast, bacteria, cyanobacteria, were tested for zinc, nickel, chromium, strontium, etc. removal from wastewater.
 Employees of the SNAAAR FLNP JINR Inga Zinicovscaia, Nikita Yuhin, Dmitrii Grozdov, Konstantin Vergel
Employees of the SNAAAR FLNP JINR Inga Zinicovscaia, Nikita Yuhin, Dmitrii Grozdov, Konstantin Vergel
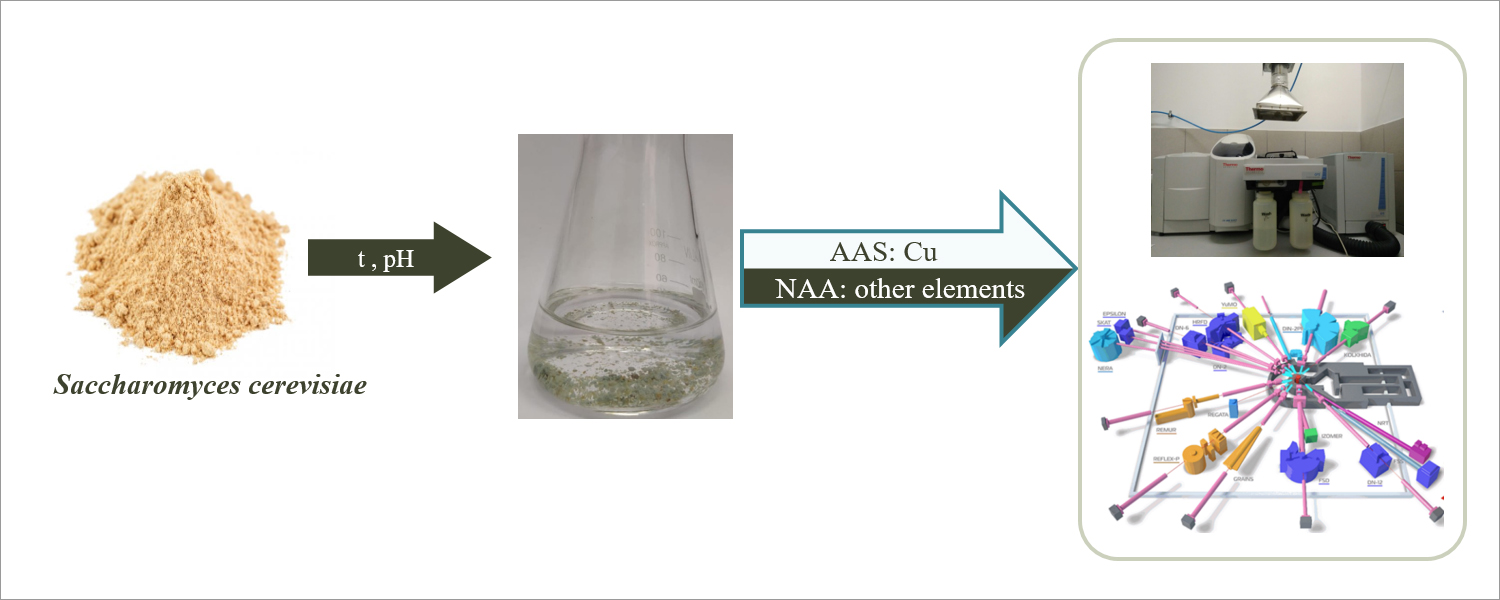
Based on the performed experiments yeast Saccharomyces cerevisiae was considered as one of the most efficient biosorbents. These microorganisms are extensively used in beverage and food production and are therefore available in large quantities at a low cost as a by-product of fermentation processes. Moreover, they are safe for humans and possess high sorption capacity for heavy metals. Using yeast, a series of experiments on model systems and wastewater of electrochemical processes were performed. To assess the efficiency of metal removal by biosorbents scientists used neutron activation analysis at the fast-pulsed reactor IBR-2 of FLNP, sensitive technique for qualitative and quantitative determination of metal content. Atomic absorption spectrometry was used to assess copper uptake by biosorbents.
To assess the efficiency of adsorption of zinc, nickel, strontium and barium in model effluents, the effect of parameters, such as time of contact, temperature, zinc concentration, pH, was studied. According to obtained results, at a pH range of 3.0-6.0, which is optimal for metal removal, in 15-45 minutes 45-100% of metal ions can be removed from wastewater (depending on the metal). At increase of zinc ions concentration in solution, the biosorbent more actively removed zinc, without affecting the sorption of other metal ions.
 Equipment used for experiments— orbital shaker Unimax 1010
Equipment used for experiments— orbital shaker Unimax 1010
The study of the effect of time on the rate of metal removal allows calculating the kinetics of the sorption, that is, the mechanism and rate of the processes allowing to understand the nature of biosorption – chemical or physical. Surface adsorption, chemisorption, and ion exchange were found to be the main mechanisms of metal removal from solution.
In addition, the scientists evaluated the effect of sorbent dosage and pH on the sorption process in experiments with industrial effluents (Fig. 1).
Thus, at a biosorbent concentration of 10 g/L, by varying pH, it was possible to reduce the concentration of copper, barium, strontium, and nickel ions below the maximum permissible concentration. The maximum efficiency of zinc ions removal was achieved in two stages at pH 6.0 and contact time of 60 min: first, by adding the biosorbent at a dosage of 20 g/L, and then adding 10 g/L of yeast biomass to the effluent obtained after the first stage of treatment (Fig. 2). The additional stage for zinc ions removal was necessary due to higher concentration of this metal compared to other pollutants.
 Fig. 2 Removal of zinc ions from industrial effluent at different sorbent dosage: (а) first stage of treatment and (b) second stage of treatment
Fig. 2 Removal of zinc ions from industrial effluent at different sorbent dosage: (а) first stage of treatment and (b) second stage of treatment
Thus, the scientists have shown that yeast cells of Saccharomyces cerevisiae are an excellent candidate for treatment of complex industrial effluents containing zinc and other heavy metal ions, and the sorption efficiency, first of all, is dependent on the pH values and the dosage of the sorbent.
The results of the study were published in the paper: Inga Zinicovscaia, Nikita Yushin, Daler Abdusamadzoda, Dmitrii Grozdov and Margarita Shvetsova. Efficient Removal of Metals from Synthetic and Real Galvanic Zinc–Containing Effluents by Brewer’s Yeast Saccharomyces cerevisiae. Materials 2020, 13(16), 3624; https://doi.org/10.3390/ma13163624