FLNP JINR researchers studied charge effect on morphological changes of cell membrane
News, 14 July 2023
A group of scientists at the Laboratory of Neutron Physics (FLNP JINR), in collaboration with colleagues from the Laboratory of Information Technologies, continues to study the interaction of amyloid-beta peptide (Aβ25-35) with model lipid membranes. These interactions provide a basis for an amyloid-beta theory of the etiology of Alzheimer’s disease that explains the damage of lipid membranes of brain cells caused by the influence of the amyloid-beta peptides. The latest experiment by JINR scientists has shown that previously observed morphological changes of membranes and their damage are independent of the charge existence in the system.
Since 2019, JINR scientists, supported by the RNF grant (No. 19-72-20186), have been studying the properties of cell membranes and their interactions with Aβ25-35. In 2021, during the previous experiment, JINR scientists were the first to show that interactions between Aβ25-35 and lipid membranes were a source of morphological changes of the latter under the influence of temperature. These changes then lead to the damage of membranes and the death of cells. JINR scientists studied the interaction of the peptide with model lipid membranes by means of small angle neutron scattering using the YuMO Spectrometer at the IBR-2 Reactor at FLNP JINR.
In a new experiment, already in 2022, specialists studied the influence of charge on the peptide-triggered morphological changes by introducing the anionic lipid (a lipid with a negative charge) into an uncharged model membrane. Changes to the membrane thickness and the overall membrane structure with and without incorporated Aβ25-35 were studied over a wide range of temperatures.
“Our results confirmed the data obtained during the previous experiment: model lipid systems undergo morphological reformations during the phase transition. Under the reduced temperature and during the phase transition of lipids, which form a model membrane, to the gel phase in the presence of Aβ25-35, scientists were observing the membrane damage, the decay of vesicles in bicelle-like structures (flat disc-like structures from one lipid bilayer). Without Aβ25-35, vesicles remained whole. The charge in the studied system had no effect on this process,” one of the scientists, an FLNP JINR senior researcher Oleksandr Ivankov highlighted.
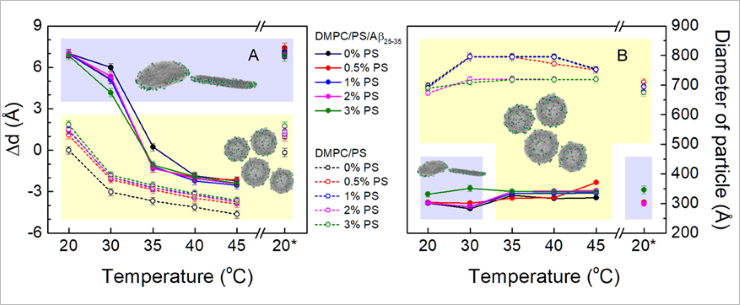 Relative change in membrane thickness Δd (A) and diameter of bicelle-like structures and single-layer vesicles (B) obtained as a result of approximation of small angle neutron scattering data
Relative change in membrane thickness Δd (A) and diameter of bicelle-like structures and single-layer vesicles (B) obtained as a result of approximation of small angle neutron scattering data
Alzheimer’s disease is a serious neurodegenerative disease characterised by the progression of dementia and complete loss of cognitive functions. Understanding the molecular basis of the etiology of the disease is necessary not only for the development of therapies, but also for proper prevention, as well as early diagnosis, which is extremely complicated by the long period of the asymptomatic stage of the disease. In this regard, the study of lipid-peptide interactions leading to global morphological changes in membranes can play a key role in understanding the causes of Alzheimer’s disease, as the scientist noted.
Publication
- Anionic lipids modulate little the reorganization effect of amyloid-beta peptide on membranes. Ivankov, O., D.R. Badreeva, E.V. Ermakova, T. Kondela, T.N. Murugova, and N. Kucerka. “Anionic Lipids Modulate Little the Reorganization Effect of Amyloid-Beta Peptide on Membranes”, General Physiology and Biophysics, vol. 42, no. 1 (2023): 59-66.