Neutron diffraction for the study of lithium-ion batteries
Publications, 05 August 2020
The article is about the application of neutron diffraction for the study of structural and microstructural transformations of electrodes while using them. The team of authors comprising of I. A. Bobrikov, A. M. Balagurov, N. Yu. Samoylova, S. V. Sumnikov, O. Yu. Ivanshina, R. N. Vasin was awarded for this scientific work with the first JINR Prize for 2019 in the section “Applied research and technology papers”.
Lithium-ion batteries (LIB) have long become an integral component of most modern portable electrical devices; they are actively introduced in automobiles as the major power source and used as energy storage devices for power plants. A large number of scientists and engineers are working to improve LIB characteristics, the main of which are energy storage capacity, charge-discharge rate, and safety of operation. This work requires a thorough understanding of all processes occurring in battery components during operation.
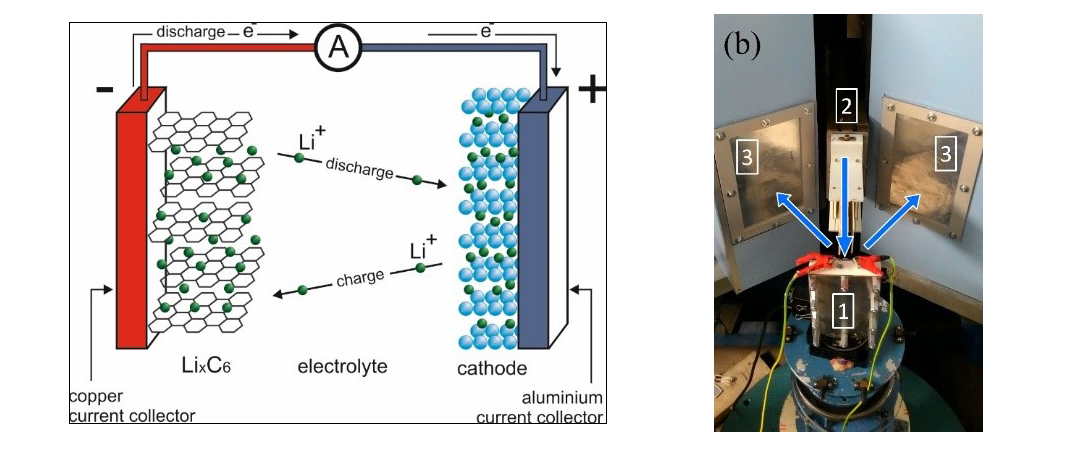
The main characteristics and principles of operation of modern lithium-ion batteries are primarily determined by the parameters of the crystal structure and elemental composition of materials of electrodes (cathode and anode), which are the key components of such batteries (Fig. 1). The cathode is usually a complex oxide based on lithium and transition metals (Mn, Fe, Co, Ni), while the anode in the vast majority of cases is synthetic graphite of various grades with different microstructures (lithium titanate oxide or lithium metal is more rarely used). All these materials have a crystal structure, which is studied using short-wave (X-ray, synchrotron or neutron) diffraction methods, among which neutron diffraction has proved to be a unique technique for studying electrode materials due to its high penetration power and the fact that the neutron scattering cross-section randomly depends on the element’s atomic number. The specific features of the neutron scattering cross-section for chemical elements make it possible to successfully study structures containing light elements (e.g., Li, O), and distinguish the position of elements with close atomic numbers (e.g., Mn, Fe, Co, Ni). The high penetration power of neutrons allows studying structural and microstructural transformations of electrodes directly in a real device and in real-time during its operation (Fig. 2).
Our research group was one of the first to conduct neutron diffraction experiments to study the operation of LIB electrodes in real-time. The first diffraction experiments were carried out on one of the neutron diffractometers of the IBR-2 pulsed reactor in 2012; preliminary results were reported in “JINR News” in 2013 and in 2014, first full-length scientific papers were published [1, 2]. Since then, in our investigations, we have managed to cover all the main types of structures used in LIB cathode and anode materials, and to establish cooperation with a large number of scientific groups in Russia and abroad that are engaged in the synthesis of new electrode materials (Moscow State University, Saratov State University, the Institute of Solid State Chemistry and Mechanochemistry of the Siberian Branch of the Russian Academy of Sciences, the Skolkovo Innovation Center, the National Tsing Hua University). Based on the results of the performed investigations, we have studied in detail the transformation of the crystal structure and microstructure of olivine-like cathode materials during their electrochemical cycling, and identified the cause of the positive effect of addition of ultra-small amounts of vanadium on the capacity of these cathodes [1]; the sequence of structural transitions in the anode material between the lithiated phases of graphite in the process of intercalation-deintercalation of lithium was studied [1, 3, 4]; the phase stability of a number of layered cathode materials was investigated, the numerical simulation of a number of structural processes was performed [5], and the anomalous behaviour of the structure of these electrodes during cycling was explained [4].
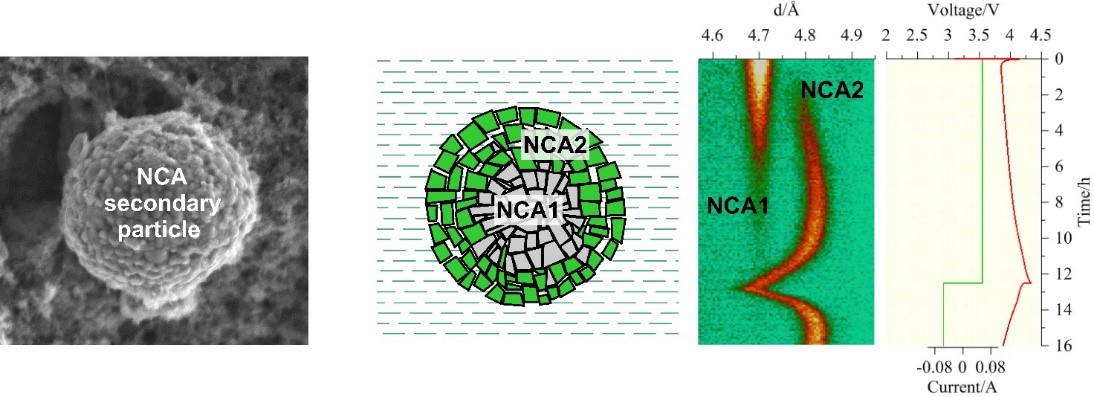
In one of our recent research studies aimed at investigating electrodes in real time, we continued to investigate the phase stability of the cathode material LiNi0,8Co0,15Al0,05O2 during charge-discharge with a focus on the behavior of the cathode structure in the first cycle [6]. For these purposes we designed specialized electrochemical cells and organized a special laboratory for local production of LIB electrodes for research. As a result of the study, for the first time using neutrons, a two-phase structural state was recorded that occurs during the first charge cycle of a layered cathode material and disappears when it is further discharged, and no longer appears in subsequent cycles. We managed to explain this effect due to the fact that the study was performed with a series of electrodes rolled with different degrees of compaction. It turned out that the cause of the phase separation is determined by the morphology of particles of the cathode material obtained by co-precipitation ― sphere-like framboids (secondary particles, sizes ~ 5-10 μm) composed of crystallites (primary particles, sizes ~ 0.2-0.5 μm). Such kind of microstructure and low ionic conductivity of the cathode material lead to gradual activation of the cathode primary particles, which manifests itself on the diffraction pattern as the coexistence of two structural phases (Fig. 3). A high compaction degree of the electrodes (more than 25% of the initial thickness) results in partial destruction of framboids, thus reducing the effect of phase separation.
Summing up, it should be said that the performed work allowed us not only to obtain a number of important scientific results, but also to demonstrate the exceptional capabilities of the neutron diffraction method for real-time investigation of structural and microstructural processes in such complex multicomponent objects as modern electrochemical current sources. The success of diffraction studies stimulated further interest in studying processes in lithium-ion batteries at IBR-2 of FLNP using other neutron scattering techniques, including reflectometry and small-angle scattering.
References
- Bobrikov I. A., Balagurov A. M., Chih-Wei Hu, Chih-Hao Lee, Deleg S., Balagurov D. A. Structural Evolution in LiFePO4-Based Battery Materials: In-Situ and Ex-Situ Time-of-Flight Neutron Diffraction Study // Journal of Power Sources. 2014. V. 258. P. 356‒364.
- Balagurov A. M., Bobrikov I. A., Samoylova N. Y., Drozhzhin O. A., Antipov E. V. Neutron Scattering for Analysis of Processes in Lithium-Ion Batteries // Russian Chemical Reviews. 2014. V. 83, No. 12. P. 1120–1134.
- Bobrikov I. A., Samoylova N. Yu., Balagurov D. A., Ivanshina O. Yu., Drozhzhin O. A., Balagurov A. M. Neutron Diffraction Analysis of Structural Transformations in Lithium-Ion Batteries // Russian Journal of Electrochemistry. 2017. V. 53, No. 2. P. 178–186.
- Bobrikov I. A., Samoylova N. Yu., Sumnikov S. V., Ivanshina O. Yu., Vasin R. N., Beskrovnyi A. I., Balagurov A. M. In-Situ Time-of-Fight Neutron Diffraction Study of the Structure Evolution of Electrode Materials in Commercial Battery with LiNi0,8Co0,15Al0,05O2 Cathode // Journal of Power Sources. 2017. V. 372. P. 74‒81.
- Eremin R. A., Zolotarev P. N., Ivanshina O. Yu., Bobrikov I. A. // Li(Ni,Co,Al)O2 Cathode Delithiation: A Combination of Topological Analysis, Density Functional Theory, Neutron Diffraction, and Machine Learning Techniques // J. Phys. Chem. C. 2017. V. 121, No. 51. P. 28293‒28305.
- Bobrikov I. A., Samoylova N. Yu., Ivanshina O. Yu., Sumnikov S. V., Vasin R. N., Korneeva E. A., Balagurov A. M. Abnormal Phase-Separated State of LixNi0.8Co0.15Al0.05O2 in the First Charge: Effect of Electrode Compaction // Electrochim. Acta. 2018. V. 265. P. 726.
Fig. 1. а) Illustration of the operation of Li-ion batteries. The charging process is shown — transition of lithium from the anode (lithium graphite or metallic lithium) through a separator (organic electrolyte) to the cathode (usually LiCoO2, LiFePO4 and other materials). Copper and aluminium are usually used as current collectors. b) Electrochemical cell (1) during the experiment at the HRFD diffractometer. (2) neutron collimator, (3) entrance windows of neutron detectors. Incident and scattered neutrons are shown by arrows. Figures 1‒3 are taken from [6
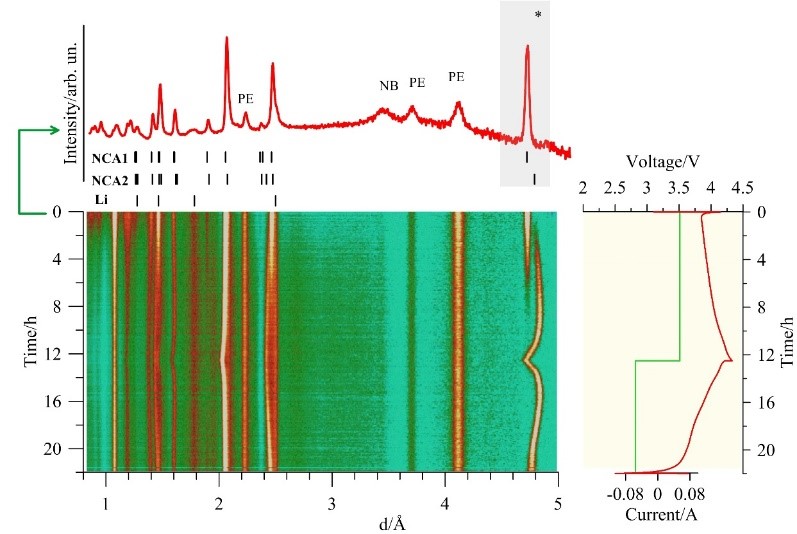
Fig. 2. During charge-discharge of the electrochemical cell (or battery), the crystal structure of the electrodes undergoes changes, which, in turn, lead to a change in the position and intensity of the diffraction peaks. Top: example of a diffraction pattern with the main contribution by cathode material (NCA) and lithium anode. PE ― separator, NB ― boron nitride. Bottom: 2D evolution of this diffraction pattern during charge-discharge. The voltage and current values during charge-discharge are shown in the graph on the right
Fig. 3. Illustration of the structural phase separation process observed in the layered cathode LiNi1-x-yCoxAlyO2 (NCA) during the first charge. From left to right: SEM image of a cathode particle; schematic representation of this particle during the first charge, where NCA1 and NCA2 are non-activated and activated structural phases; their manifestation in the evolution of the neutron diffraction peak from the cathode material