Collaboration of JINR Laboratories in search for origins of Alzheimer’s disease
News, 15 February 2021
Neutrons and molecular simulations: scrutinizing the neural membranes damage caused by amyloid beta peptide
According to statistics, every tenth elderly person has a significant decrease in cognitive functions of the brain. And very often this is the cause of Alzheimer’s disease, i.e. a neurodegenerative disease characterized by the death of nerve cells in the brain. The disease is currently poorly studied and belongs to the category of incurable ones. Since 2019, the FLNP, LIT and LRB collaboration, supported by the RNF grant (No. 19-72-20186), has been working to study the properties of cell membranes and cell interactions in order to understand and study the mechanisms of Alzheimer’s disease.
Alzheimer’s disease is a neurodegenerative brain disease, first described by the German psychiatrist Alois Alzheimer in 1907, and the most common form of dementia (60% – 70% of all cases), characterized by an impairment of cognitive and physical abilities of a person. There are about 50 million people with dementia all over the world and this number is projected to be about 75 million by 2030 and 131 million by 2050 [1].
At the present time there is no clear understanding of the causes of Alzheimer’s disease. However, there are several hypotheses that try to explain the origin of this disease. The most relevant of these appear to be the hereditary hypothesis [2], the cholinergic hypothesis [3], the amyloid hypothesis [4], and the tau hypothesis [5].
The amyloid hypothesis, which was first postulated in 1991, is associated with the amyloid beta peptide, formed as a result of proteolytic cleavage of the APP (amyloid beta precursor) glycoprotein, which as a rule is always present in the membranes of neurons and other cells. Under certain conditions, the amyloid beta oligomer can undergo an incorrect conformational rearrangement leading to the transition of “normal” soluble peptides into a toxic conformation, while causing the formation of filamentous aggregates – insoluble rigid fibrils of large sizes, that are a sign of the disease. However, the toxic effect, which has a destructive effect on the nerve cells of the brain, is possessed already by isolated misfolded peptides born in the cell membrane (Fig. 1) [6].
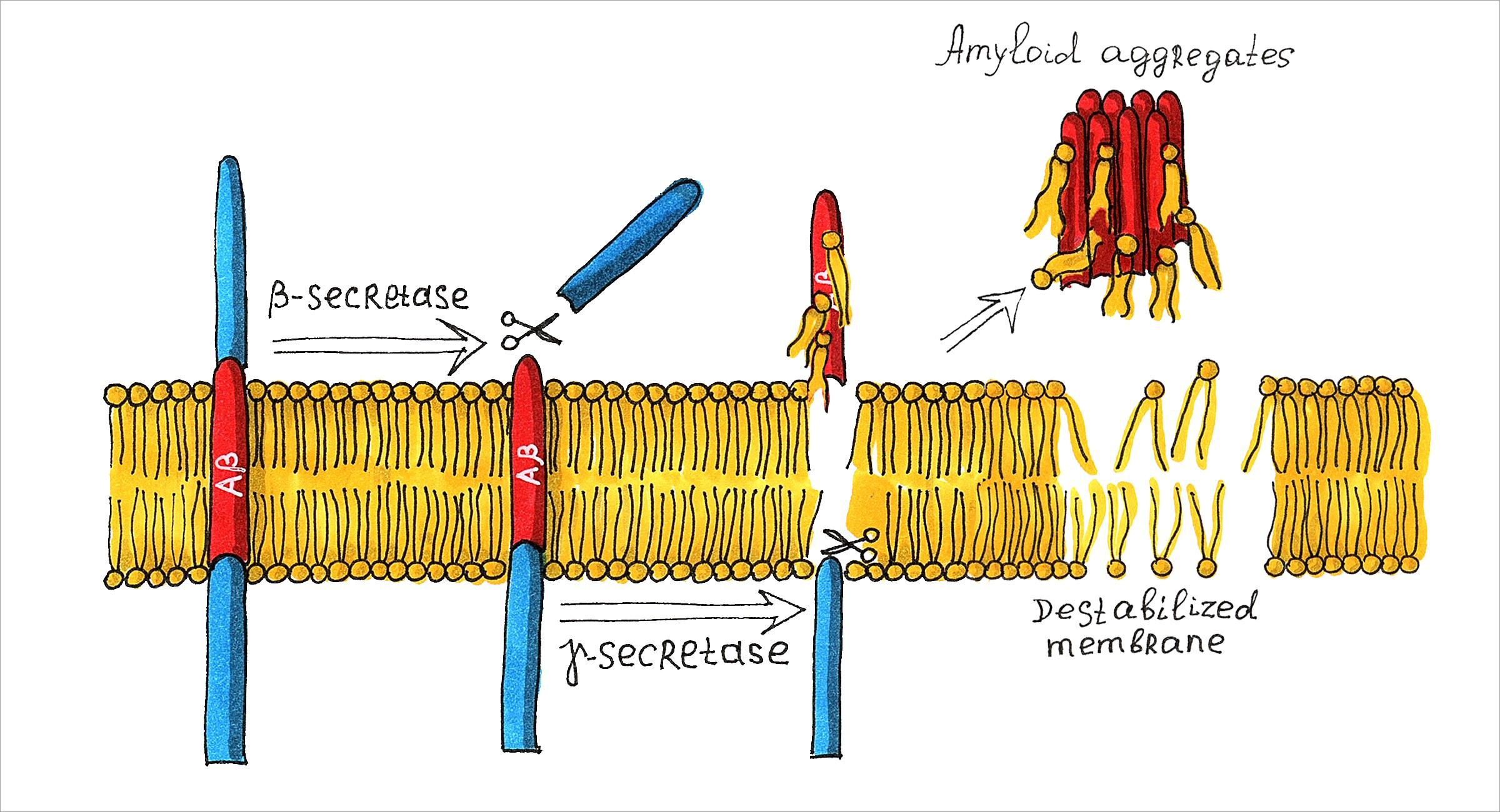 Fig. 1. The sketch of the amyloid hypothesis of Alzheimer’s disease. As a result of the amyloid precursor protein cleavage by β-secretase and γ-secretase via the amyloidogenic pathway, insoluble Aβ fragments prone to aggregation are formed. In the case of misfolding, they have toxic effects.
Fig. 1. The sketch of the amyloid hypothesis of Alzheimer’s disease. As a result of the amyloid precursor protein cleavage by β-secretase and γ-secretase via the amyloidogenic pathway, insoluble Aβ fragments prone to aggregation are formed. In the case of misfolding, they have toxic effects.
It is assumed that an important role at the initial stage of Alzheimer’s disease is played by the Aβ peptide interactions with cell membranes. These interactions are regulated by the membrane composition, which in turn correlates with its structural dynamic and elasto-mechanical properties [7]. It is obvious that the investigation of the influence of the membrane composition and properties is a necessary condition for a complete understanding of the triggering mechanisms of Alzheimer’s disease. Since 2019, a collaboration among FLNP, LIT, and LRB with the support of the Russian Science Foundation (grant № 19-72-20186) has been working along this direction. By using the experimental and theoretical research methods like small-angle neutron and X-ray scattering and inelastic neutron scattering, carried out at the YuMO and NERA instruments of the IBR-2 reactor, and Rigaku spectrometer (MIPT), calorimetry, densitometry and molecular dynamics simulations on the heterogeneous HybriLIT platform, we shed light on the influence of structural (bilayer thickness, lateral surface area at the water-lipid interface, level of hydration) and dynamic (elasticity, diffusion) membrane properties, and/or chemical lipid properties (composition and charge of the head, length and unsaturation of hydrocarbon tails) on peptide-membrane interactions.
Currently, model systems of various biological membranes in the presence of ions, cholesterol, melatonin, and amyloid beta peptide have been studied by small-angle neutron scattering and molecular dynamics [8-10]. It was found that metal divalent cations interact with the cell membrane, binding to the polar heads of bilayered lipids. This changes the thickness and structure of the membrane and affects the conformations and functions of various proteins embedded in it.
The effect of cholesterol also causes a perceptible change to the membrane thickness, associated with the fact that cholesterol orders the lipid hydrocarbon tails and, therefore, causes their elongation, which increases the thickness and the viscosity of the membrane. The effect of the melatonin, which on the contrary reduces the membrane thickness, is associated with the disordering of lipid tails. Melatonin molecules are localized in the area of phospholipid heads and lead to an increase in the distance between them. This increases the conformational space of lipid tails and therefore decreases the bilayer order parameter, which causes a decrease in thickness and viscosity of the membrane.
In addition, studying the Aβ interaction with model lipid membranes loaded with cholesterol and melatonin by small-angle neutron scattering and neutron diffraction methods, it was revealed that the peptide is preferably localized in the head groups of membrane lipids. In the case of peptide insertion into a model membrane, it is mainly located in the regions under the lipid head groups, as well as at the water-lipid interface, while increasing the membrane thickness. When cholesterol is added to the membrane, the peptide location does not change much. This effect can be explained by the fact that cholesterol is localized in the region of the tail groups and does not interact with the peptide. The melatonin addition to the membrane, which has a thinning effect, on the other hand affects the peptide position, presumably shifting it towards the water-lipid interface. Visualization of the molecular dynamics results also showed the tendency of peptide molecules to localize in the lipid head group, as well as an increase in the membrane thickness (Fig. 2). A significant conformational change in peptide molecules was also demonstrated – the transition from alpha helices to disordered secondary structures.
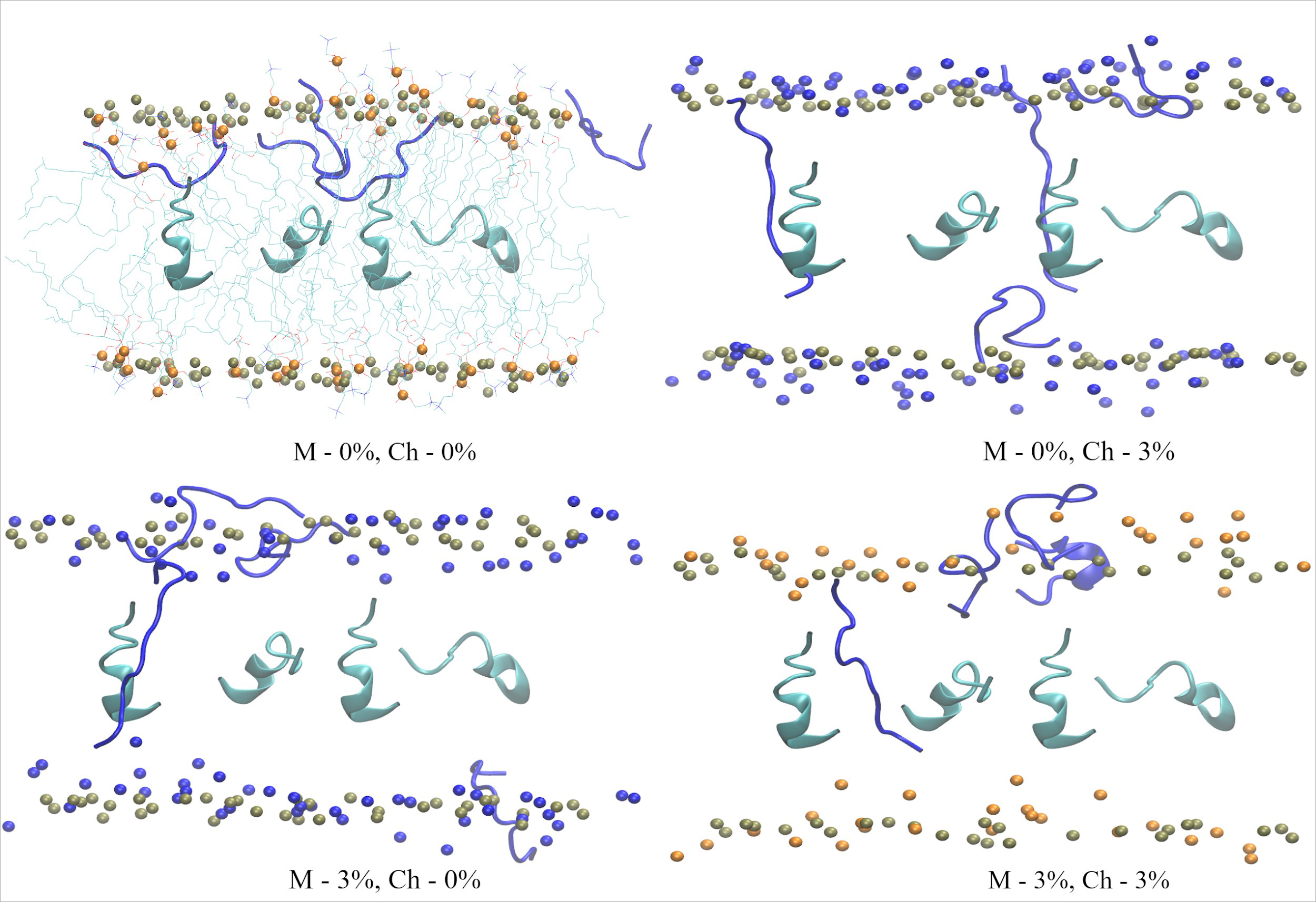 Fig. 2. The MD simulation snapshots demonstrating the location of the amyloid beta peptide interacting with lipid bilayers loaded with cholesterol (Ch) and melatonin (M) at various concentrations. The peptides at the beginning of the simulation are turquoise, and at the end of the simulation – blue.
Fig. 2. The MD simulation snapshots demonstrating the location of the amyloid beta peptide interacting with lipid bilayers loaded with cholesterol (Ch) and melatonin (M) at various concentrations. The peptides at the beginning of the simulation are turquoise, and at the end of the simulation – blue.
By the differential scanning calorimetry and densitometry methods, it was corroborated that the cholesterol addition and the peptide embedding into the membrane increase the phase transition temperature, which correlates with the lipid packing density increase and the membrane rigidization. The addition of melatonin to the membrane decreases the main phase transition temperature and the lipid packing density, and makes the membrane more fluid.
The influence of the membrane thermodynamic state on the Aβ insertion into the membrane was also studied. Based on the type of the change in the membrane thickness, it can be concluded that, in the ordered gel phase, the peptide incorporation has a greater effect on the system and the membrane thickness. In the case of the disordered liquid crystal phase, incorporation of the peptide also affects the membrane thickness, but this effect is much lower. At the same time, the effect of the temperature influence on the rearrangement of the membrane shape (change in the spherical vesicle size and the formation of nanodisks) was first discovered, which is directly linked to the peptide presence in the membrane. This process is completely reversible, and in the peptide absence, the size of the vesicles remains unchanged, which unambiguously indicates the destructive action of the peptide (Fig. 3).
 Fig. 3. The small-angle neutron scattering curves demonstrating the destructive effect of amyloid beta peptides on the model membrane. The shape of the membrane changes with the temperature and in the peptide presence from the large vesicles to the vesicles of small sizes and nanodiscs.
Fig. 3. The small-angle neutron scattering curves demonstrating the destructive effect of amyloid beta peptides on the model membrane. The shape of the membrane changes with the temperature and in the peptide presence from the large vesicles to the vesicles of small sizes and nanodiscs.
The information being obtained during this research on correlations between the structure and functions of membranes is obviously of interest to the applied and pharmaceutical research that relates to studies of intercellular communication, protein transport, and regulation of cholesterol capture and transport. For example, the obtained results demonstrate the key role of membrane viscosity for the peptide incorporation into it, namely, a significant decrease in the effect of peptide insertion into the membrane that is loaded with melatonin, compared to a neat membrane or a membrane loaded with cholesterol. The results can be used in further studies of the pathogenicity of this amyloid, since they may provide some insight into the molecular mechanism of the protective function of melatonin in Alzheimer’s disease.
With the development of modern experimental techniques, the improvement of neutron and X-ray radiation sources, the development of additional effective detection tools and an increase in the power of high performance computing clusters, a new cycle of the Alzheimer’s disease investigation has begun, which occupies a major place in biomedical research. Of course, the study of this disease is far from being complete. What has been obtained already is a number of important results, such as hypotheses describing the origin of the disease, models describing the interactions leading to the onset of the disease, and the most importantly, there are proposals for possible methods of combating the disease. Perhaps, utilizing the neutron scattering in a closer look at the Aβ peptide-membrane interactions will be part of the final solution in defeating Alzheimer’s disease.
D. R. Badreeva, P. Hrubovčák, E. B. Dushanov, E. V. Ermakova, O. I. Ivankov,
T. Kondela, A. I. Kuklin, S. A. Kurakin, T. N. Murugova, V. V. Skoi,
D. V. Soloviov, Kh. T. Kholmurodov and N. Kučerka
References
- Prince M., Wimo A., Guerchet M., Ali G. C., Wu Y. T., Prina M. (2015). The global impact of dementia: an analysis of prevalence, incidence, cost and trends. World Alzheimer Report, 2015.
- Reitz C., Mayeux R. (2014). Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochemical pharmacology, 88(4), 640-651.
- Francis P. T., Palmer A. M., Snape M., Wilcock G. K. (1999). The cholinergic hypothesis of Alzheimer’s disease: a review of progress. Journal of Neurology, Neurosurgery & Psychiatry, 66(2), 137-147.
- Hardy J., Allsop D. (1991). Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends in pharmacological sciences, 12, 383-388.
- Mudher A., Lovestone S. (2002). Alzheimer’s disease – do tauists and baptists finally shake hands?. Trends in neurosciences, 25(1), 22-26.
- Hardy J., Selkoe D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. science, 297(5580), 353-356.
- Niu Z., Zhang Z., Zhao W., Yang, J. (2018). Interactions between amyloid β peptide and lipid membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1860(9), 1663-1669.
- Kurakin S. A., Ermakova E. V., Ivankov O. I., Smerdova S. G., Kučerka N. (2021). The effect of divalent ions on the bilayer structure of dimyristoylphosphatidylcholine vesicles. Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques, 3.
- Murugova T., Ivankov O., Ermakova E., Kondela T., Hrubovčák P., Skoi V., Kuklin A., Kučerka N. (2020). Structural changes introduced by cholesterol and melatonin to the model membranes mimicking preclinical conformational diseases. General physiology and biophysics, 39(2), 135-144.
- Ivankov O. I., Ermakova E. V., Murugova T. N., Badreeva D. R., Dushanov E., Kondela T., Kholmurodov Kh., Kuklin A., Kučerka N. (2020). Interactions in the model membranes mimicking preclinical conformational diseases. In Advances in Biomembranes and Lipid Self-Assembly (Vol. 31, pp. 185-214). Academic Press.