Nanoscale structure of electrochemical interfaces for lithium power sources
Publications, 24 June 2021
The second JINR prize for 2020 in the nomination “Applied physics research” was awarded to a joint team from FLNP JINR and the Department of Chemistry of Moscow State University, consisting of: M.V. Avdeev, V.I. Petrenko, I.V. Gapon, O.I. Ivankov, E.E. Ushakova, Y.N. Kosiachkin, D.M. Itkis, L.V. Yashina, A.A. Rulev, T.K. Zakharchenko. The prize was awarded to the series of studies “Nanoscale structure of planar and developed electrochemical interfaces for lithium power sources by neutron scattering”.
The current rapid development of electric transport, robotics, as well as the miniaturization and enhancement of the functionality of portable electronic devices, require more advanced electrochemical energy storage devices. To date, the highest specific energy storage (up to 240 W h / kg) is achieved in lithium-ion batteries, which are based on the ability of electrode materials to include (intercalate) and extract (deintercalate) lithium ions during battery charging/discharging. Further ways of a significant increase in the specific energy of electrochemical sources are associated with non-intercalating type lithium energy storage devices, including lithium-ion sources with a metal anode, as well as lithium-oxygen cells based on carbon cathodes with a record theoretical specific energy storage (600 Wh / kg and higher). The development of appropriate power supplies, however, encounters difficulties associated with the fact that a number of undesirable processes occur at the electrochemical interfaces, leading to a decrease in the efficiency of the power sources, as well as causing problems in their safety. In particular, the reason for this is the formation and growth of mesoscopic structures: (i) needle-like formations (often called “dendrites”) during the electrodeposition of metallic lithium during charging; (ii) layers of amorphous and crystalline discharge products and supercrystalline structures in the case of lithium-oxygen cells. In this regard, the development of experimental approaches that would make it possible to describe the structure of inhomogeneities at electrochemical interfaces, primarily for the nanoscale (1-100 nm), which determines the evolution of the interface during the storage device operation, is of current interest. For this purpose, the effectiveness of the methods using the scattering of thermal neutrons was demonstrated for studying planar interfaces (by neutron reflectometry) and developed interfaces (by small-angle neutron scattering). The high penetrating power of neutrons makes it possible to study complex systems with interfaces hidden for many methods. The presence of a liquid component in the electrolyte made it possible to use extremely efficiently the contrast variation in neutron scattering based on hydrogen / deuterium isotopic substitution.
The presented series of studies is devoted to the diagnostics and investigation of electrochemical interfaces for advanced lithium energy storage devices. It covers experimental structural research carried out in the Frank Laboratory of Neutron Physics of JINR at the IBR-2 pulsed reactor in the period of 2017-2020 using neutron reflectometry (at the GRAINS reflectometer) and small-angle neutron scattering (at the YuMO instrument). As a result, the relationship between the microstructure of interfaces in materials used in electrochemical cells and the characteristics of the cells is shown under different conditions. Based on the structural information revealed, recommendations are formulated on the composition and synthesis of materials to improve the capacity, conductivity and safety of functioning of new generation lithium-ion and lithium-oxygen energy storage cells. For example, in relation to lithium-oxygen storage devices, the considered transition to electrolytes with high solvatation of lithium ions in the liquid base radically changes the process of deposition of electrochemical reaction products, which makes it possible to more than double the current limit on the capacity of this type of storage devices. Another example is solid ceramic-type electrolytes, for which the use of special homogenizers of the structure was considered, which ultimately makes it possible to increase the conductivity of ceramic membranes by more than five times. Electrochemical cells specially designed and manufactured by the research team were used for studying model interfaces of the ‘solid electrode – liquid electrolyte’ type. The data on neutron scattering were analyzed together with the extensive electrochemical characterization performed at the Department of Chemistry of Lomonosov Moscow State University.
It was shown that neutron reflectometry can be effectively used to characterize various modes of deposition of lithium on metal electrodes [1-4]. A two-stage nature of deposition was found, when, at the first stage, a dense lithium-enriched layer is formed on the electrode surface from the products of the chemical interaction of lithium ions with the solvent, and at the second stage, a transition layer starts to form, which corresponds to the onset of the appearance of large mesoscopic inhomogeneities (needle-like structures). When modifying the electrolyte by adding a non-electrically active additive as a measure against such kind of inhomogeneities, there is a strong suppression of growth and a significant change in the composition of the near-surface layer.
 Fig. 1. Analysis of specular reflection of neutrons from planar interfaces “metal electrode – liquid electrolyte”, which change during the operation of the electrochemical cell. The growth of a lithium-enriched layer during the deposition and its modification by introducing a special additive into the electrolyte composition to suppress parasitic formations on the electrode surface, are investigated. To enhance the reflection, hydrogen-deuterium isotopic substitution in the electrolyte is used. Experimental data were obtained at the GRAINS reflectometer of the IBR-2 reactor, JINR. Figure is adapted from [2].
Fig. 1. Analysis of specular reflection of neutrons from planar interfaces “metal electrode – liquid electrolyte”, which change during the operation of the electrochemical cell. The growth of a lithium-enriched layer during the deposition and its modification by introducing a special additive into the electrolyte composition to suppress parasitic formations on the electrode surface, are investigated. To enhance the reflection, hydrogen-deuterium isotopic substitution in the electrolyte is used. Experimental data were obtained at the GRAINS reflectometer of the IBR-2 reactor, JINR. Figure is adapted from [2].
From the data of small-angle neutron scattering [5], the blocking of oxygen transport pathways in wetted cathodes in lithium-oxygen cells occurs both due to the passivation of the inner pore surface inside the carbon grains of the cathode material, as well as due to the growth of supramolecular structures in the intergranular space. The high solvation of lithium ions in the liquid base of the electrolyte determines the rapid diffusion of lithium-containing complexes formed during electrochemical reactions and promotes the predominant release of lithium peroxide into the intergranular space. A prime example of such a solvent is dimethyl sulfoxide. When it is used, the fraction of filled nanopores in carbon grains does not depend on the discharge current; lithium peroxide mesocrystals are also formed throughout the entire volume of the material.
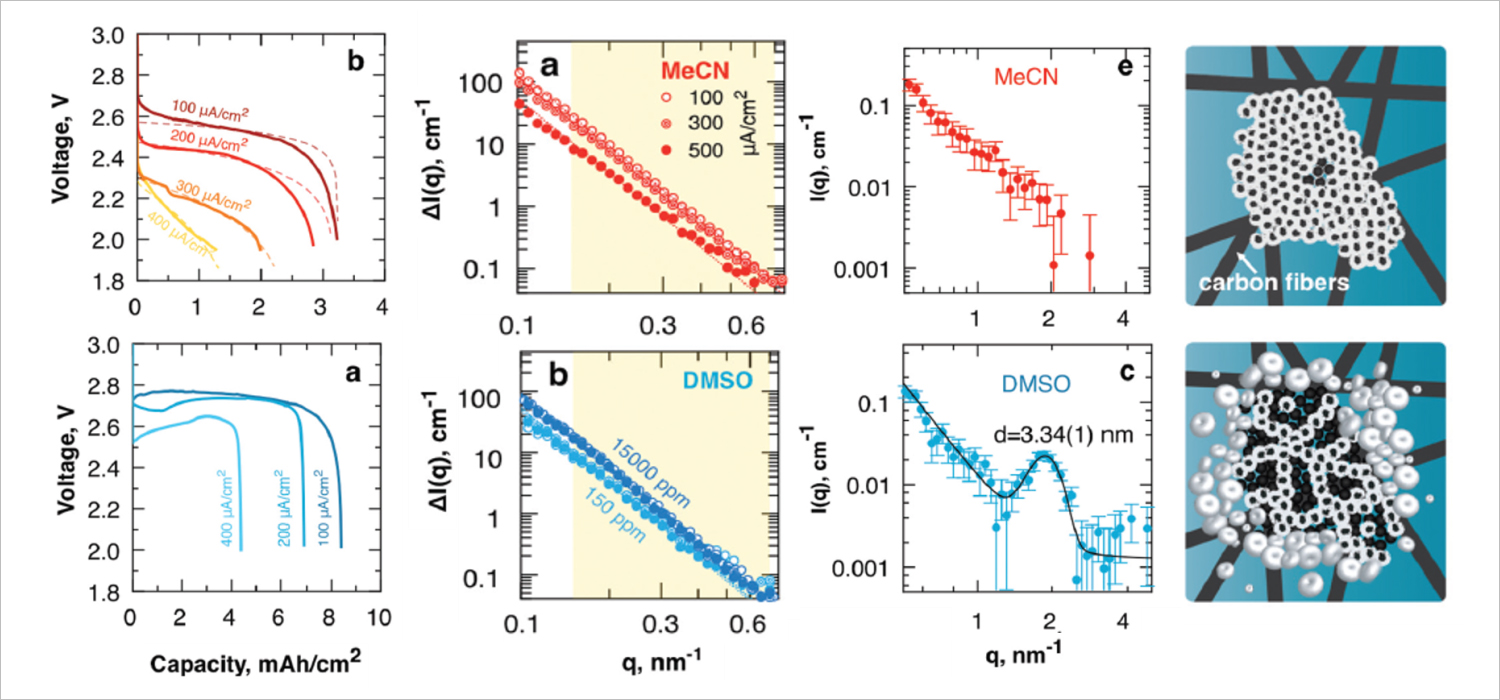 Fig. 2. Evolution of lithium-oxygen model cells for different liquid electrolyte bases: the top row is for acetonitrile (MeCN); the bottom row is for dimethyl sulfoxide (DMSO). From left to right are shown: voltage profiles during discharging at different rates; the initial region of the curves of small-angle neutron scattering during discharge at different rates; the far region of the curves of small-angle neutron scattering after discharge at different rates; schematic representation of the deposition process of lithium peroxide in the carbon grain of the cathode. The neutron scattering data were obtained at the YuMO instrument of the IBR-2 reactor, JINR. Figure adapted from [5].
Fig. 2. Evolution of lithium-oxygen model cells for different liquid electrolyte bases: the top row is for acetonitrile (MeCN); the bottom row is for dimethyl sulfoxide (DMSO). From left to right are shown: voltage profiles during discharging at different rates; the initial region of the curves of small-angle neutron scattering during discharge at different rates; the far region of the curves of small-angle neutron scattering after discharge at different rates; schematic representation of the deposition process of lithium peroxide in the carbon grain of the cathode. The neutron scattering data were obtained at the YuMO instrument of the IBR-2 reactor, JINR. Figure adapted from [5].
To ensure the structural homogeneity of lithium-conducting LAGP membranes (NASICON type) for lithium-oxygen cells during crystallization by annealing, it was proposed to use a homogenizing additive in the form of an yttrium compound, which significantly improved both mechanical and electrochemical properties of the membranes. With the help of small-angle neutron scattering [6,7], it was possible to explain the mechanism for improving the conductivity of the membranes when using the additive: the modification of the material leads to a more uniform shape of crystal grains, as well as to a decrease in porosity; as a result, an increase in intergranular contacts takes place.
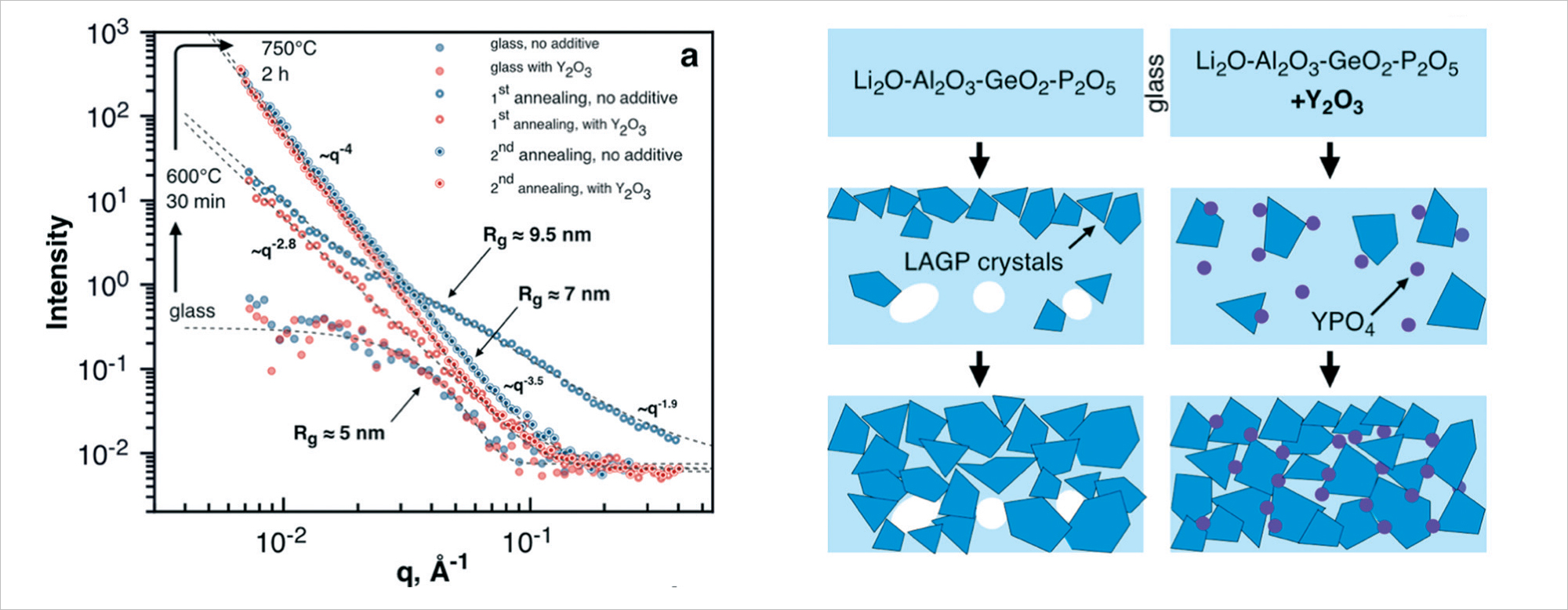 Fig. 3. Small-angle neutron scattering for different stages of the synthesis of lithium-conducting LAGP membranes of the NASICON type upon crystallization of pure LAGP and with the addition of a homogenizer (yttrium oxide) to the initial composition. The corresponding evolutions of the membrane structure at the nanoscale (1-100 nm) are schematically shown. The neutron scattering data were obtained at the YuMO instrument of the IBR-2 reactor. Figure is adapted from [6].
Fig. 3. Small-angle neutron scattering for different stages of the synthesis of lithium-conducting LAGP membranes of the NASICON type upon crystallization of pure LAGP and with the addition of a homogenizer (yttrium oxide) to the initial composition. The corresponding evolutions of the membrane structure at the nanoscale (1-100 nm) are schematically shown. The neutron scattering data were obtained at the YuMO instrument of the IBR-2 reactor. Figure is adapted from [6].
References
[1] M.V. Avdeev, A.A. Rulev, V.I. Bodnarchuk, E.E. Ushakova, V.I. Petrenko, I.V. Gapon, O.V. Tomchuk, V.A. Matveev, N.A. Pleshanov, E.Yu. Kataev, L.V. Yashina, D.M. Itkis, Monitoring of lithium plating by neutron reflectometry. Applied Surface Science 424 (2017) 378-382.
[2] M.V. Avdeev, A.A. Rulev, E.E. Ushakova, Ye.N. Kosiachkin, V.I. Petrenko, I.V. Gapon, E.Yu. Kataev, V.A. Matveev, L.V. Yashina, D.M. Itkis, On nanoscale structure of planar electrochemical interfaces metal/liquid lithium ion electrolyte by neutron reflectometry, Applied Surface Science 486 (2019) 287-291.
[3] Petrenko V.I., Kosiachkin Ye.N., Bulavin L.A., Avdeev M.V., On Enhancement of the Adsorption-Layer Effect at the Metallic Electrode−Liquid Electrolyte Interface in Specular Neutron Reflectometry Experiments. Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques 12(4) (2018) 651-657.
[4] Petrenko V.I., Kosiachkin Ye.N., Bulavin L.A., Avdeev M.V., Optimization of the Initial Interface Configuration for In-Situ Neutron Reflectometry Experiments. Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques 14(4) (2020) 215–219.
[5] T.K. Zakharchenko, M.V. Avdeev, A.V. Sergeev, A.V. Chertovich, O.I.Ivankov, V.I. Petrenko, Y. Shao-Horn, L.V. Yashina, D.M. Itkis, Small-angle neutron scattering studies of pore filling in carbon electrodes: mechanisms limiting lithium–air battery capacity. Nanoscale 11 (2019) 6838-6845.
[6] V.A. Vizgalov, T. Nestler, L.A. Trusov, I.A. Bobrikov, O.I. Ivankov, M.V. Avdeev, M. Motylenko, E. Brendler, A. Vyalikh, D.C. Meyer, D.M. Itkis, Enhancing lithium-ion conductivity in NASICON glass-ceramics by adding yttria. CrystEngComm 20 (2018) 1375-1382.
[7] V.A. Vizgalov, T. Nestler, A. Vyalikh, I.A. Bobrikov, O.I. Ivankov, V. Petrenko, M.V. Avdeev, L.V. Yashina, D.M. Itkis, The role of glass crystallization processes in preparation of high Li-conductive NASICON-type ceramics. CrystEngComm 21 (2019) 3106-3115.